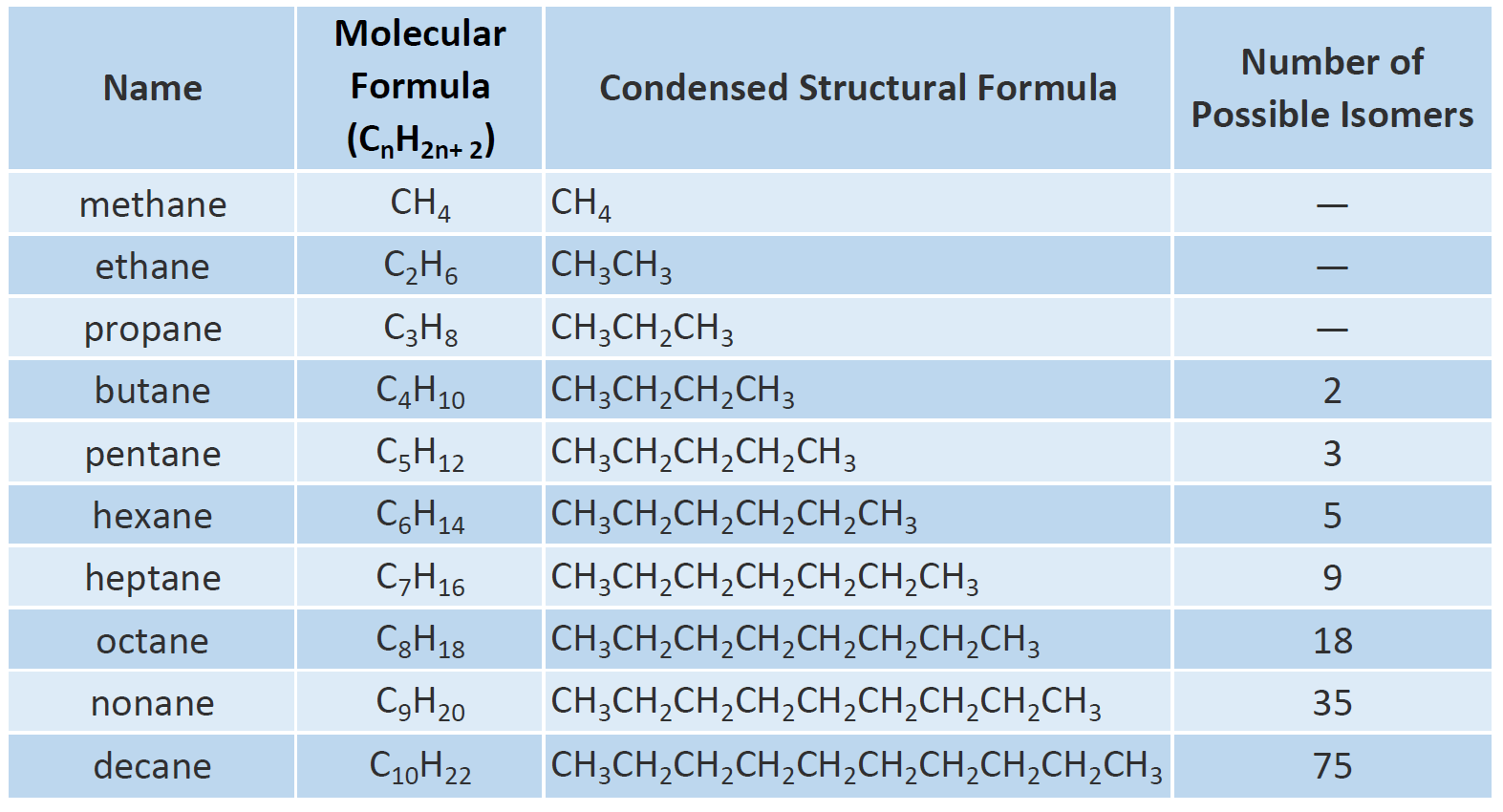
General Formula of Alkane
Contain 10 carbon atoms. For example the chemical compound ethylene is known to have an odour that is quite sweet and musty.

What Is The Formula Of Alkane Quora
A Find the longest continuous carbon chain and select the appropriate alkane name from Table 1.
. Nomenclature of substituted alkanes can further be understood by considering the following problem. Remember that carbon must have four bonds oxygen must have two bonds and hydrogen can only have one bond. Hydrocarbons are the principal constituents of petroleum and natural gas.
The simplest alkane is methane with the formula ofCH4. If a simple unbranched alkane is converted to a cycloalkane two hydrogen atoms one from each end of the chain must be lost. The following steps are taken in naming an alkane with a branched chain.
Thinking about this fact is it possible to have the carboxylic acid -textCOOH group in a. If more than one stereoisomer of product is formed draw both. Our online organic chemistry trivia quizzes can be adapted to suit your requirements for taking some of the top organic chemistry quizzes.
Hence the 2C 2 in the formula is the total number of hydrogen atoms that should be bound to the saturated carbon atoms and H is the number of hydrogen atoms that are actually present in the compound. The general formula for the alkanes is C n H 2n 2 where n is the number of carbon atoms in the molecule. In general olefins or more specifically alkenes are known to have relatively stronger odours than their alkane counterparts.
General formula for alkyl groups is CnH2n1 Unit 12. The most common alcohol known as ethanol is used in alcoholic drinks fuel gasoline a preservative for biological specimens and a solvent for paints and drugs. These are called respectively butane and 2-methylpropane.
Side chains are not included in the carbon count b Name all of the side chains carbon chains attached to the longest chain and list them in alphabetical order. Has a functional group. For each extra.
In other words a saturated form of a hydrocarbon will have the maximum number of hydrogen atoms in an acyclic alkane form. A comprehensive database of more than 30 organic chemistry quizzes online test your knowledge with organic chemistry quiz questions. In naming alcohols the suffix of the name -textol is.
General Structure or Formula. Some types of oils and waxes are the examples of alkanes with many carbon atoms number. The last yellow shaded column gives the general formula for a cycloalkane of any size.
The formula for an alkyne is variable as well. Alcohols have the same general formula as alkanes but the structure of alcohol functional group is textOH called the hydroxyl group. There is no limit of how much carbons can be tied together.
It also can be more than 10 carbon atoms. Number the carbons in the longest carbon chain Important. An alkyne with only one triple bond has the formula CnH2n-2.
Problem 132 Write structur es of dif fer ent isomeric alkyl gr oups corr esponding to the molecular for mula. The general formula for alkanes is CnH2n 2. Get free list of Chemistry formulas online at ClearIITMedical.
Each triple bond means two fewer hydrogens than a corresponding alkane. Alkanes have the general chemical formula C n H 2n2The alkanes range in complexity from. It consists of four hydrogen atoms and one carbon atom and is the simplest alkane.
What is the general formula for this series. The nomenclature becomes more complex if the alkane branches. If the molecule is not an alkane ie.
Methane is the simplest of saturated hydrocarbons with a chemical formula CH 4. Pentan-1-yl is an example of a name by this method and is synonymous with pentyl from the previous guideline. Let us recall the general rules for nomenclature already discussed in Unit 12.
Hence the general formula for. The more general method omits only the terminal e of the substituent name but requires explicit numbering of each yl prefix even at position 1 except for -ylidyne which as a triple bond must terminate the substituent carbon chain. When natural methane reaches the surface of the atmosphere is called atmospheric methane and can be found under the seafloor as well as below the ground.
For example C 4 H 10 could be either of these two different molecules. Draw a structural formula for the substitution product of the reaction shown below. The carbon atoms join together to form the framework of the compound and the hydrogen atoms attach to them in many different configurations.
Naming structural isomers of alkanes. Common examples of such. Solve out any chemical reaction using these formulas only from ClearIITMedical.
If a simple unbranched alkane is converted to a cycloalkane two hydrogen atoms one from each end of the chain must be lost. Hydrocarbon any of a class of organic chemical compounds composed only of the elements carbon C and hydrogen H. Strained alkenes in particular are known to possess extremely solid unpleasant odours.
Hence the general formula for. F CH3 Na OČCH3 CHCO2H Use the wedgehash bond tools to indicate stereochemistry where it exists. The reason that the value of.
The last yellow shaded column gives the general formula for a cycloalkane of any size. Separate multiple products using the sign from the drop-down menu. Decane is an alkane.
They serve as fuels. This means that there are two or more different structural formulae that you can draw for each molecular formula. In organic chemistry an alkane or paraffin a historical trivial name that also has other meanings is an acyclic saturated hydrocarbonIn other words an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carboncarbon bonds are single.

Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry
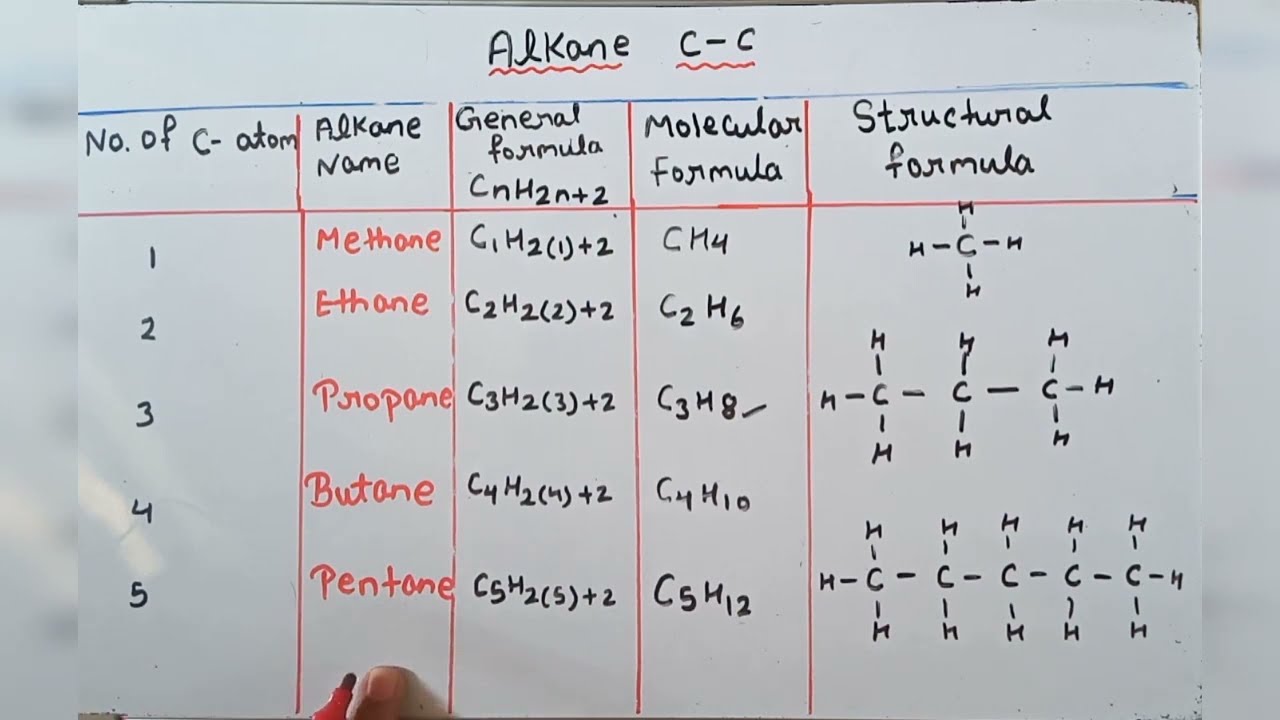
Alkane Molecular Structural General Formula Youtube

Question Video Applying The General Formula For Alkane Chemical Formulas Nagwa

Class 10 General Formula Of Alkane Alkene Alkyne Tx Academy Youtube
Comments
Post a Comment